
Bullous Pemphigoid Clinical Trial
Bullous Pemphigoid Clinical Trial
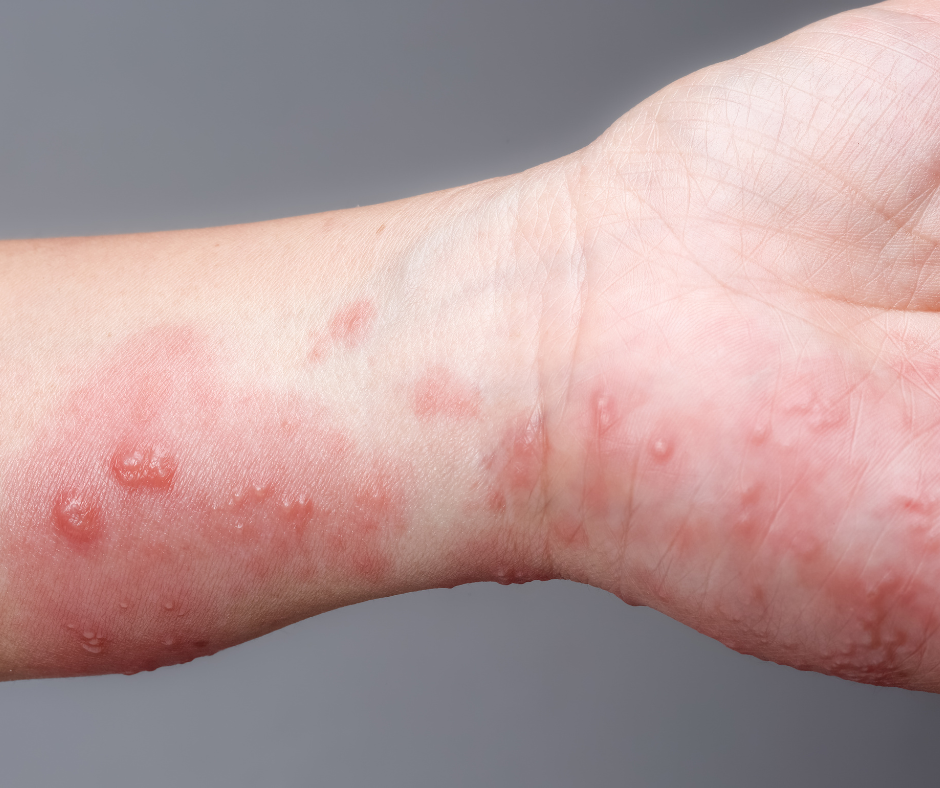
Patients with Bullous Pemphigoid, A rare skin condition causes large, fluid-filled blisters, will be enrolled in this study to evaluate new treatments for this skin condition. Clinical trial participants will be monitored closely by medical professionals for the duration of the study. The goal of this Bullous Pemphigoid, trial is to develop better understanding of Bullous Pemphigoid and develop safe and effective therapies.
Study Snapshot
Duration
Up to 60 weeks depending on study
Incentive
Subject compensation and travel assistance may be available. Study medication, study related exams, and lab tests will be provided at no cost.
Have questions about our bullous pemphigoid clinical trial?
For general questions regarding our clinical trials, please contact our team at clinicaltrials@clearlyderm.com or fill out the contact form below.
Frequently Asked Questions
Click or Press on the Questions to See the Answers.
A clinical trial is a research study conducted with human volunteers to test the effectiveness and safety of new drugs, devices, or interventions. Our Clearlyderm clinical trials focus on skin conditions such as psoriasis, acne, rosacea, or atopic dermatitis (eczema).
To enroll in one of our clinical trials, contact our office. You will need to review and complete informed consent documents and complete screening procedures prior to participation.
Like any medical treatment, potential risks exist. However, participants are informed of these risks prior to starting the clinical trials and receive monitoring and follow-up care throughout the trial duration if selected to participate.
Bullous pemphigoid (BP) is an autoimmune disease that occurs when the immune system attacks and destroys healthy tissue. This leads to large, fluid-filled blisters on the skin.
The goal of this trial is to study the safety and efficacy of a drug that we are hopeful will be used as a treatment for BP in the future. This particular drug is already used to treat various other autoimmune diseases.
Around 45 weeks
Schedule an appointment
Looking to visit a dermatologist? Click the button below to schedule an appointment with our team of skin experts.




